Quality Management Module
Quality Management Module
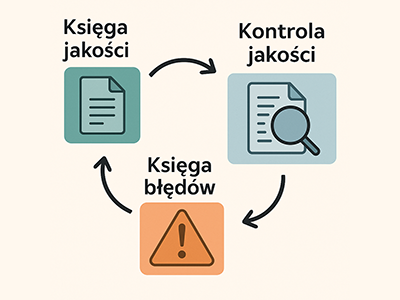
The LISPAT Quality Management Module is a comprehensive tool designed to maintain and continuously improve work standards within the pathology lab. It seamlessly integrates documentation, error tracking, and result re-verification into a single, cohesive ecosystem.
- Quality Manual – a central, always up-to-date repository of procedures, instructions, and policies (with version control and built-in PDF preview).
- Error Register – a record of pre-analytical, transport, analytical, and interpretive errors, partly auto-detected, partly reported manually.
- Quality Control – random statistical reviews, internal/external consultations, and post-operative re-evaluations.
These elements work together automatically: quality control findings populate the Error Register, and reports from both areas can be combined into trend analyses and corrective actions. This allows teams to quickly identify root causes, document compliance (e.g. with Polish Society of Pathologists guidelines), and effectively improve quality and safety across the board.
Quality Manual
The Quality Manual module in LISPAT is an integral part of the Quality Management Module, designed for defining and maintaining a complete documentation system – including the Quality Manual itself, procedures, and work instructions. It supports a clear, tree-based structure where the manual sits at the top level, with related procedures and instructions below.
Each item in the structure can have a linked PDF stored on the file server, accessible from any workstation in the network. Documents can be updated repeatedly – each new version automatically archives the previous one. Archived versions are visibly marked with strikethrough and a clear yellow-bannered comment to prevent confusion with the current version.
A built-in PDF viewer allows for instant document preview without downloading. A double-click is enough to display the content. All users have read access to the Quality Manual, but only authorized personnel can upload or update documents.
Having the Quality Manual in this form offers a huge organizational advantage. The documentation is always up to date, centrally located, and accessible within seconds. Staff can be confident they are following current procedures and instructions, reducing error risk and streamlining internal and external audits.
This solution enhances consistency across the facility, ensures compliance with quality standards, and simplifies meeting regulatory requirements – all contributing to higher patient safety and better team performance.
Error Register
The LISPAT Error Register combines automatic and manual mechanisms to record and analyze mistakes occurring at various stages of the diagnostic process. Its purpose is not only to collect data, but to support root cause analysis and error prevention.
Types of errors recorded include:
- Pre-analytical errors – e.g. missing data on referrals, mismatches in material quantity or labeling, improper fixation.
- Transport errors – e.g. poor transport conditions, fixation errors, inappropriate delivery methods.
- Analytical errors – e.g. equipment failure, contamination, material loss during processing.
- Interpretive errors – when a second reviewer gives a different opinion than the first.
Some errors are automatically detected (e.g. missing patient name on referral), while others are entered manually by staff. Data from the Error Register can be used for statistical reports and to implement corrective measures.
Quality Control
Quality control in LISPAT includes various forms of result re-evaluation:
- Statistical control – the system randomly selects a set percentage of cases (e.g. 10% of negative gynecological cytology) for re-review.
- Internal or external consultation – materials are forwarded to another pathologist upon request by the primary reviewer.
- Post-operative histopathological review – conducted after surgery to compare with the intraoperative assessment.
Re-evaluation results are compared to the originals. In case of discrepancies, the system logs an error: either regular (minor differences) or critical (those affecting treatment decisions). The quality control and error tracking systems are fully integrated, enabling a complete feedback loop and comprehensive reporting.